It is no secret that there is a lack of diversity in clinical research. With fewer than five percent of all adults diagnosed with cancer participating in trials, the adverse effects of this disparity are magnified when it comes to the treatment and care of minority patients.
Clinical trials form the cornerstone of new treatments and drug approvals. Their results guide future policies and decision-making for treating lung cancer patients. However, barriers to minority participation in research mean these trials often fail to represent the entire community these treatments are intended to serve. A study in JAMA Oncology found that 76% of the participants in cancer trials were white, while 18% were Black, 3% were Asian, and 6% were Hispanic. Compared with the proportion of U.S. cancer patients, white participants represented 98% of the expected value. Blacks and Hispanics represented only 22% and 44%, respectively. (Asian individuals were overrepresented at 438% of their expected values in clinical trials.)
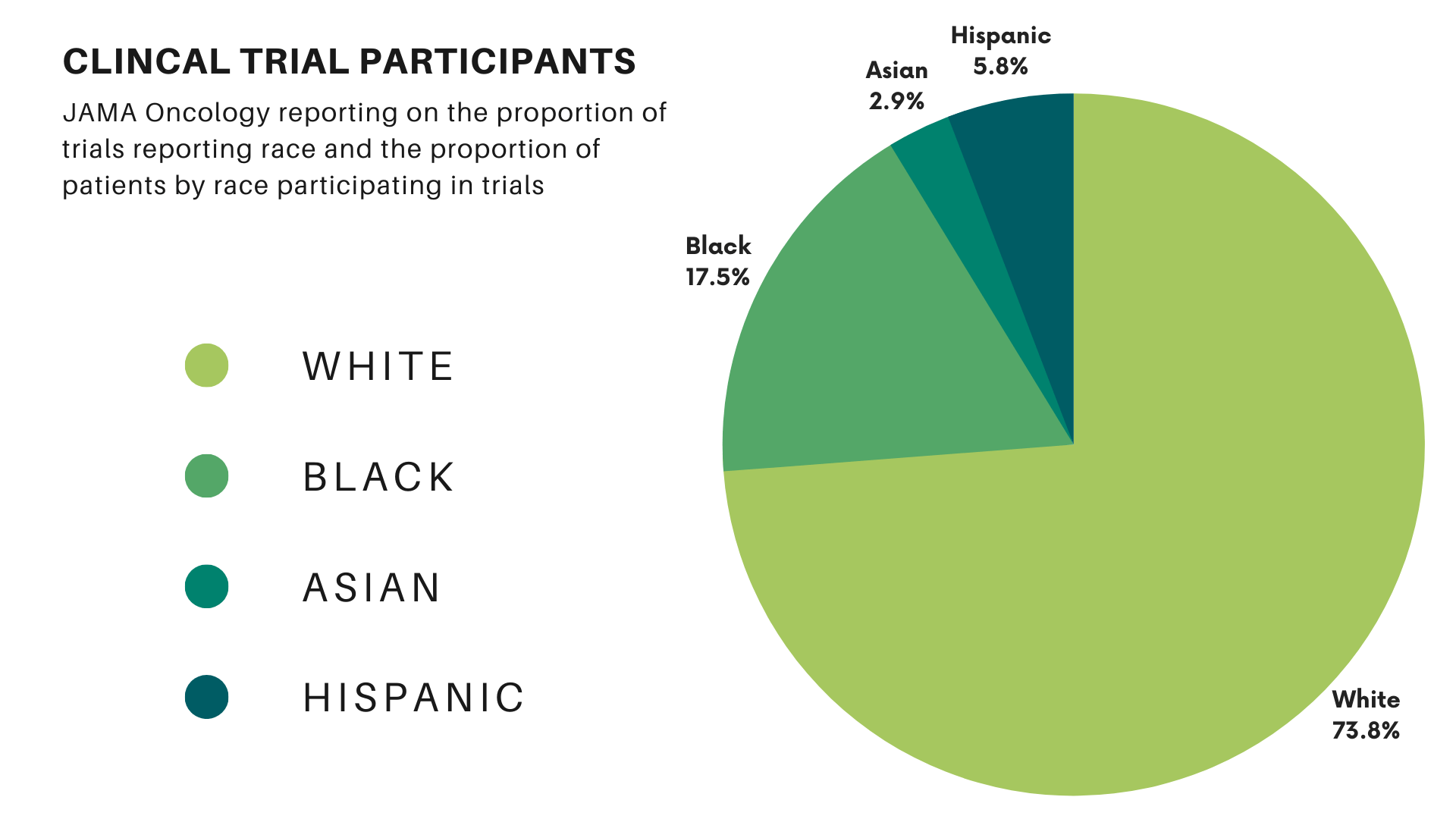
Barriers to Minority Participation in Clinical Trials
The disparities in clinical trials significantly impact the efficacy of treatment for underrepresented groups. This impact will only increase as the trend of personalized medicine continues and treatments like immunotherapy are explicitly tailored to one’s genotype. Without sufficient data regarding a patient group, doctors have limited ability to predict how they may respond to treatment. For example, a study published in 2017 found that some African Americans have lung tumors with a different gene expression than those found in white patients. This could impact the efficacy of specific therapies.
More Inclusion Leads to More Effective Treatments
Medical treatments, like any advancement, tend to benefit most those that have easy access to them. Generally this means those who traditionally have the time, connections, and finances to take advantage of new treatments and participate in clinical trials. In the U.S., patients who enroll in cancer treatment trials tend to be younger, male, and white. This lack of diversity in clinical trials can lead to a cycle where new treatments are most effective for the most represented group. The results are not generalizable or misapplied to certain populations and perpetuate health disparities in the lung cancer continuum. (Health disparities are experienced by groups with greater social or economic obstacles. This is based on characteristics historically linked to discrimination or exclusion.) The International Association for the Study of Lung Cancer employs the “iceberg” framework to demonstrate how disparities can extend past racial and ethnic lines.
Underrepresented groups may feel discouraged from participating in trials as the hope for breakthrough treatments for them feels ever out of reach. But there are reasons these groups should feel hopeful and push to participate in clinical trials. These reasons include receiving better individual care and benefitting your community.
A History of Mistrust Leads to Lack of Diversity in Clinical Trials
History includes many instances of racial disparities in healthcare, where minority groups were left out of or outright denied adequate treatment. In the late 19th century, Chinese immigrants in San Francisco were routinely denied care at the City and County Hospital. When an outbreak of the bubonic plague occurred, little was done by state officials to protect the Chinese residents in the city. Not until Texas threatened to cut off trade did the state spring into action. California finally addressed the unsanitary conditions in the neighborhood caused by inadequate care and unethical landlords.
In 1932, the infamous Tuskegee Syphilis Experiment advertised a “free blood test” and “free treatment” to poor Black men. Unbeknownst to them or their families, these men were injected with syphilis. They were never treated. All so researchers could observe the effects of the disease. The study was halted in 1972 when it was deemed ethically unjustified.
Community Activists Respond
In the 1980s, in the face of government silence and inaction in response to the AIDS crisis, members of the affected communities banded together to provide care for those falling ill. Activists formed the Gay Men’s Health Crisis in 1982 and the AIDS Coalition to Unleash Power (ACT UP) in 1987, among other groups.
These are only a few examples. It is no wonder distrust of the medical establishment still exists in some of these communities. The COVID-19 pandemic has continued to highlight some of these problematic trends and touched on historical factors contributing to this distrust.
Historical and social factors serve as additional barriers to treatment. Even if the circumstance is passed, the weight of its memory remains.
Other Barriers to Minority Participation in Clinical Trials
The lack of trust and willingness to participant on the part of minority patients is one major factor in the lack of diversity in clinical trials. Another factor is the pharmaceutical industry’s involvement. The drive for companies to find expedited pathways for drug approval has given them less incentive to seek out minority participants, which often requires extra time. There’s also the lack of public trust in pharmaceutical companies in general. Finally, doctors often do not have a clear plan of engagement to discuss options with patients or may simply lack the time.
Reasons to Participate in Lung Cancer Trials
Despite these factors, today several safeguards exist to protect participants in clinical trials and rebuild trust. The Belmont Report established its three basic principles for research in 1976:
- Respect for persons—respecting the individual’s autonomy and the right to choose the treatment they receive.
- Beneficence—offering the most benefit with the least amount of risk
- Justice—attempting to share the benefits and burdens equally
Researchers must adhere to these values in clinical trials. All researchers owe participants the same duty of care they owe to their own patients.
Additionally, federal initiatives have sought to increase the participation of minority patients in medical trials. The National Institutes of Health (NIH) Revitalization Act of 1993 is one such initiative. The FDA Safety and Innovation Act of 2012 is another. However, these measures have had limited success, especially in increasing enrollment for Black and Hispanics. As the population is projected to be less than half non-Hispanic white by 2045, increasing diversity in clinical trials becomes ever more crucial.
Minority patients are more likely to face barriers when it comes to participating in clinical trials. These include:
- a lack of resources to locate trials,
- difficulty accessing medical facilities where research usually takes place,
- indirect costs such as loss of income, and
- a lack of trust in the medical establishment.
However, the lack of diversity in cancer trials can keep individual minority patients from receiving adequate care. It can also keep them from benefiting from scientific advances. This hinders innovation for future research.
Receive the best individual care
All participants in lung cancer clinical trials receive, at minimum, the best standard of care available. That is, no participant will receive a placebo or be left untreated when there is an effective treatment available. Additionally, while a majority of Black, Latinx, LGBTQ+, and people of lower socioeconomic status believe they are mistreated or misunderstood by the medical establishment, trial participants can receive more access to doctors and nurses and a greater chance for their voice to be heard. Plus, the trial’s sponsor covers research costs, like drugs, diagnostic tests, treatments, and doctor visits related to the study.
Clinical trials often offer the most cutting-edge treatment available. A lung cancer patient participating in them can receive better care than routine treatment. In fact, a trial might provide treatment that is otherwise completely unavailable.
Lasting Benefits for You and Your Community
The racial disparities in clinical trials for lung cancer do more than just impact a single study. It can have ramifications for the efficacy of patient treatment and safety for an entire group. As mentioned previously, the trend in personalized medicine makes accounting for specific genotypes increasingly important. Biological and genetic differences factor into the efficacy of drugs. Without sufficient diversity in trials, those factors will be missed for minority groups. For example, this may be especially important for the Hispanic community. Studies show that ancestry may play a significant role in their NSCLC tumor mutation profiles and impact treatment.
Lack of minority participation in clinical trials also makes it difficult to identify early safety signals for adverse events for patients in these groups. For example, 24% of lung cancer cases in women and 17% of lung cancer cases in men are in individuals who have never smoked. “Despite this,” Dr. Moorthi states in a discussion on lung cancer research disparities, “the majority of tumor genome-wide sequencing efforts in lung cancer have been focused on patient cohorts with a smoking history.” Other group members may benefit from the increased awareness your participation lends. The greater the number of participants from a group, the better able medical researchers will be to tailor treatments to patients and swiftly address any concerns.
Dr. Raymond Osarogiagbon recommended action in his speech to LCFA during World Lung Cancer Day 2021. He said patients need to “begin to hold people accountable for the care they provide.” Dr. O notes that there is nothing new in the disparities to access to healthcare. Rather than focus on these points, he urges patients to speak for themselves and their fellow humans to combat the cynicism found in some medical professionals. As mentioned above, one of the main contributors to a lack of diversity in clinical trials is that many providers do not feel they have the time or information to discuss clinical trials in detail with patients and address any concerns.
Another step you can take is to seek out a clinical trial or patient navigator. They are nurses or lay people who work to find trial options. These navigators can also aid you in negotiating eligibility issues, unpacking complex medical and trial information. They offer support with other strains of treatment. Sometimes doctors may unintentionally neglect to offer trials to patients they assume will not follow the treatment protocol. The presence of a trial navigator can add extra support for the patient and reassure the practitioner.
Learn More About Clinical Trials
Finally, you can learn more about clinical trials. The National Cancer Institute and the National Library of Medicine (clinicaltrials.gov) provide databases of publicly and privately funded studies from around the U.S. and the world. And the NCI Community Oncology Research Program (NCORP) is a national network that connects members of a community with cancer clinical trials and care delivery studies.
The lack of diversity in clinical trials has lasting effects on members of minority groups. This is especially true when it comes to receiving adequate healthcare. All participants in a clinical trial receive at least the base standard. And, they often can take full advantage of the latest medical advancements for the treatment of lung cancer. Participation leads to benefit for you and future lung cancer patients like you.
